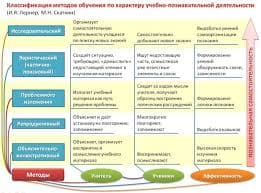
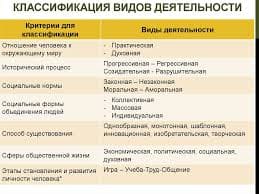
(physiological mechanisms)
| Respiratory acidosis | - the main role belongs to the renal mechanism | ||||||||
| Non respiratory acidosis | - the main role belongs to the respiratory mechanism | ||||||||
| Respiratory alkalosis | - a main role belongs to the renal mechanism | ||||||||
| Non respiratory alkalosis | - the main role belongs to the respiratory mechanism and renal. | ||||||||
| The task № 2. | Solve clinical cases | ||||||||
| Parameters of acid-base balance, blood electrolytes, etc. biochemical parameters of blood and urine | norm | Problems | |||||||
| №1 | №2 | №3 | №4 | №5 | №6 | ||||
| рН of arterial blood | 7,35-7,45 | 7,27 | 7,28 | 7,30 | 7,29 | 7,48 | 7,42 | ||
| рСО2 (кПА) | 4,8-6,2 | 3,9 | 4,1 | 7,5 | 3,9 | 3,2 | 6,8 | ||
| Standard Hydrocarbonate (mmol/l) | 21-25 | 18,0 | 18,5 | 26,0 | 17,0 | 20,0 | 28,0 | ||
| Buffer excess (mmol/l) | -2,4..+2,3 | -5 | -5 | +1,3 | -4 | -3 | +6 | ||
| Sodium of plasma (mmol/l) | 134-169 | ||||||||
| Potassium of plasma (mmol/l) | 3,8-5,2 | 5,2 | 5,7 | 5,3 | 5,8 | 4,0 | 3,8 | ||
| Cl- of plasma (mmol/l) | 95-100 | ||||||||
| Urea of blood (mmol/l) | 3,33-8,32 | 4,6 | 2,1 | 5,25 | 22,4 | 4,6 | 6,22 | ||
| Ammonia of plasma (mmol/l) | 7,14-21,42 | 16,5 | 14,1 | 25,5 | 15,3 | 16,8 | |||
| Ketone bodies (mmol/l) | up to 5,2 | 7,2 | 5,6 | 4,0 | 5,2 | 4,2 | 3,5 | ||
| Glucose of blood (mmol/l) | 4,44-6,66 | 8,8 | 4,45 | 4,3 | 6,1 | 5,8 | 5,24 | ||
| Lactic acid of blood (mmol/l) | 0,33-0,78 | 0,8 | 3,0 | 0,85 | 0,62 | 0,42 | 0,34 | ||
| Bilirubin of plasma (total) (mmol/l) | 1,7-20,5 | 1,9 | 10,0 | 16,38 | 1,29 | 12,7 | 16,5 | ||
| Diuresis (L/day) | 0,6-1,6 | 2,6 | 1,6 | 1,5 | 0,3 | 1,8 | 1,8 | ||
| рН of urine | Sub acidic | 4,6 | 5,2 | 5,4 | 7,4 | 7,9 | 8,0 | ||
| Titration acidity of urine (ml of 0,1 M NaOH/day) | 70-100 | ||||||||
| Ammonia of urine (g/day) | 0,5-1,0 | 2,4 | 1,1 | 1,0 | 0,03 | 0,22 | 0,25 | ||
| Acetone of urine | no | is | is | no | no | no | no | ||
ALGORITHM of the decision of problems on ABB disturbances
|
|
|
1. Estimate рН of arterial blood.
2. Analyze: I. рСО2 of arterial blood; 2. Standard hydrocarbonate and Buffer excess.
3. Characterize reactions of ion exchange (according to blood contents of K+, Na +, CI¯).
4. Estimate the parameters of ABB and work of kidneys.
5. Analyze other biochemical parameters of blood and urine with the purpose of specification the form of ABB disorder.
Possible variants of the analysis of ABB disturbances.
Estimation рН of arterial blood.
рН characterizes a ratio of acids and the bases in blood. Norm is 7,35 - 7,45. When pH is not less than 7,35 acidosis is compensated, when it is less than 7,35 acidosis is decompensated; when pH is not less than 7,45 alkalosis is compensated, when it is more than 7,45 alkalosis is decompensated.
Estimation of рСО2
The quantity of soluble in plasma CO2 characterizes a respiratory component of the ABB. Change of this parameter can be connected with development of compensatory reactions at deviations of ABB, and also is caused by respiratory insufficiency.
Normal partial pressure of carbon dioxide is 4,8-6,2 КПа. It is less than 4,8 КПа at non respiratory acidosis and respiratory alkalosis. It is more than 6,2 КПа at respiratory acidosis and non respiratory alkalosis.
Estimationof standardhydrocarbonate (SB)
Under the international nomenclature (SB) is concentration of hydrocarbonates in plasma determined in standard conditions. The parameter reflects metabolic components of mechanisms of acid-base homeostasis.
SB in norm is 21-25 mmol/L. Reduction of SB testifies of decrease of blood hydrocarbonate (НСО3). It is possible at non respiratory acidosis and at respiratory alkalosis.
Reduction in blood of the НСО3- the main base connecting free Н + ions of blood at metabolic acidosis occurs at following situations:
1. Excess of organic acids at metabolism disorders or administration of acids.
|
|
|
2. Disorders of excretion of organic acids at renal failure (renal form of metabolic acidosis).
3. Excessive loss of hydrocarbonates from an organism.
Reduction SB at respiratory alkalosis occurs as result of compensatory reactions of the kidneys excreting НСО3- from organism for preservation of balance of the hydrocarbonate buffer.
Increase of SB testifies of accumulation of hydrocarbonates in blood:
а) At respiratory acidosis for maintenance of hydrocarbonate buffer balance; б) at metabolic alkalosis as consequence of excessive introduction of bases in an organism or loss of organic acids.
Estimation of BE (ВЕ – is buffer excess) – a parameter specifying a difference between actual content of the buffer bases and their normal value. This parameter in the best way characterizes a metabolic component of the acid-base homeostasis and its change specifies the metabolic reasons of ABB disorders. In norm ВЕ is 2,5 mmol/L.
At respiratory acidosis and alkalosis shifting of the parameter is insignificant and is caused by compensatory metabolic reactions. Negative values of the parameter testify of excess organic acids in an organism or about bases deficiency. Positive values testify of deficiency of organic acids or about excess of bases.
Reactions of ion exchange (determined by concentration of K +, Na +, Mg ++, CI¯) allow to receive the additional information about physical and chemical compensation (restoration of buffer systems) and about physiological compensation of ABB (physiological systems of an organism (mainly kidneys).
The most typical changes of ionic exchange reactions are:
At metabolic acidosis
Elevation of K+ in blood is the important attribute of metabolic acidosis (Н+ ions moves from plasma into erythrocytes and other cells in exchange for K+ ions).
Elevation of blood Na+ and Ca++ is due to shifting of Н +ions into bones in exchange for Na+ and Са ++, moving from bones into plasma.
At respiratory acidosis
Hypochlorinemia (chloric shift) – hemoglobin of erythrocytes connects chlorides of plasma instead of hydrocarbonates shifting from erythrocytes to plasma.
Elevation of К+ – hemoglobin absorbs hydrogen ions giving instead of them K+ ions to plasma.
At metabolic alkalosis
Hyperchlorinemia – chlorine leaves from erythrocytes replacing НСО3-, lost with urine as NaНСО3.
At respiratory alkalosis
Hyponatriemia is due to loss of NaНСО3 with urine. Protein buffer releases Н +
in exchange for Na+ ions.
Hyperchlorinemia is a result of shifting of chlorine from cells into plasma as compensation of НСО3 loss with urine.
The assessment of kidneys work is carried out under the analysis of titration acidity of urine, its рН, the level of excreted ammonia. Relations between these parameters are submitted in the table.
| Total excretion of Н + | 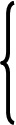 Titration acidity
(TA)
Titration acidity
(TA)
| 
| Free Н +- рН urine Connected Н + NН3+Н + | ||
1. For metabolic acidosis are characteristic high ТA, low рН of urine and significant loss of NН3 as a result of compensatory renal mechanism of ABB maintenance (acidogenesis, ammoniumgenesis). At renal failure development of metabolic acidosis is caused by disturbances of these mechanisms of acid-base balance maintenance.
2. For respiratory acidosis is typical: minor alterations of urine acidity (proton excretion is not so expressed; ammoniumgenesis begins only at significant increase of blood). Kidneys at this type of acidosis play the main role in compensatory mechanisms. Their role is to keep the ratio of acids and the bases by bicarbonate reabsorption.
3. At metabolic alkalosis ТA is low, рН is high, and losses NН3 are insignificant. Kidneys increase excretion of НСО3-, save up protons.
4. At respiratory alkalosis ТA is low, рН is high, restriction of proton removing and increase excretion of НСО3-, and Nа +.
 2015-08-13
2015-08-13 534
534