The neuroimaging intermediate phenotype is conceptually analogous to an intermediate phenotype for common complex medical disorders. It is logical to assume that genes would show stronger associations with the biological substrates contributing to risk of a disorder, with measurable quantitative traits along a pathophysiologic causal pathway, intermediate to the end complex syndrome. Intermediate phenotypes in other realms of medicine include lipid level as an intermediate phenotype for heart disease, sodium homeostasis as an intermediate phenotype for hypertension, and body mass index as an intermediate phenotype for diabetes.5,6 We favor the term “intermediate phenotype” over the more popular term “endophenotype,” though the two terms are essentially interchangeable. The term “endophenotype” (which was introduced into psychiatric genetics in the 1970s7) initially referred to a trait that is “internal” that may be discoverable by a “biochemical test or microscopic examination,” but is not external or overtlymanifest. Also, the term “endophenotype” does not emphasize the concept of intermediacy in pathogenicity. Criteria for the establishment of a neuroimaging-based intermediate phenotype for schizophrenia, as in other fields of medicine, are that the intermediate phenotype: (i) is heritable; (ii) is found with increased frequency in healthy relatives of ill probands; (iii) exists temporally before the onset of the clinical illness in the pathophysiological pathway to the emergence of the clinical syndrome. As expounded in a review by Tan et al, evidence for each of these criteria has accumulated for the syndrome of schizophrenia, with cognitive dysfunction often integral and assayable at the brain level by task-based neuroimaging intermediate phenotypes.8 Each of these criteria are consistent with the assumption that the intermediate phenotype is genetically and biologically less complex than the clinical syndrome, and that genes showing association at the syndromal level will show greater effect sizes (penetrance) on variation in intermediate phenotypes.
According to the neuroimaging genetics paradigm, to simply demonstrate that a susceptibility gene for schizophrenia impacts brain function is a necessary but not sufficient biological proof of a mechanism of susceptibility. This is because many, if not most, genes expressed in the brain, are apt to have a brain effect of some sort. A sine qua non of this proof is to show that the physiological intermediate phenotype associated with a susceptibilitygene for schizophrenia is itself linked to illness risk. To make this link, it is necessary to demonstrate that the physiological intermediate phenotype is a characteristic of individuals who are at increased genetic risk but do not manifest the clinical syndrome. The ideal samples in which to demonstrate this are unaffected relatives, eg, cotwins, siblings. This has been done for a number of brain-associated intermediate phenotypes related to increased risk for schizophrenia, including cognitive dysfunctions and neuroimaging phenotypes.9-14 Thus, the study of healthy relatives as a target population is critical for establishing the link between genetic association with clinical risk, and genetic association with biological risk. Having identified a neuroimaging phenotype related to increased genetic risk for illness, investigators can ask the question of whether genetic variation in a gene of interest maps onto the specific phenotype, as an indication of its putative neural mechanism of risk. The question arises of which population to choose to conduct this test. Neuroimaging studies of only affected subjects is confounded by illness-associated epiphenomena that are difficult to control, including smoking history, medical comorbidities, chronic illness burden, or prolonged neuroleptic exposure. This makes results in patient samples difficult to interpret, as the associations may reflect an interaction of the gene with any of these epiphenomena. Instead, the imaging genetics paradigm to test a specific gene-association hypothesis, le, the association of variation in a putative susceptibility gene and brain function linked to increased genetic risk, is best performed in healthy subjects. Healthy individuals possess common at-risk genotypes, but are not themselves symptomatic or clinically ill, thereby reducing the effect of confounding variables. This approach isolates the simple biologic effect of the genetic variation on brain function (Figure 1).
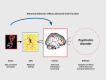
Figure 1.
 2015-07-14
2015-07-14 411
411